Dibutyl Ether: The Underestimated Solvent Star
| In the world of organic solvents, Dibutyl Ether (CAS 142-96-1) may not be as well-known as ethanol or acetone, but it plays an irreplaceable role in industrial production and laboratories. Today, we will take an in-depth look at the unique charm of this "low-key powerhouse" solvent. |
|
Dibutyl Ether's "ID Card"
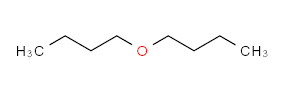
Molecular Formula: C₈H₁₈O
Molecular Weight: 130.22
Appearance: A colorless, transparent liquid with a slight ether-like odor.
Physical Parameters: It has a boiling point of 142°C, a flash point of 25°C (making it a flammable liquid), and a density of 0.77 g/cm³ (which is lighter than water).
Chemical Properties: It possesses the general chemical properties of an ether. The ether bond breaks during oxidation and nitration. Heating Dibutyl Ether with a mixture of phosphoric acid, phosphorus pentoxide, and potassium iodide forms iodobutane. It also forms chlorides when heated with titanium tetrachloride. Chlorination produces dichlorodibutyl ether, and Dibutyl Ether can also form peroxides.
Why Choose Dibutyl Ether? Three Core Advantages
Excellent Dissolving Performance
Dibutyl Ether is a medium-polarity solvent that is miscible with many organic solvents like alcohols and ethers. It can dissolve fats, resins, rubber, organic acids, esters, and alkaloids. Its unique "amphiphilic" nature allows it to dissolve non-polar substances like fats and resins while also being compatible with some polar compounds. This makes it an ideal solvent for extracting natural products (e.g., plant essential oils and alkaloids), an "invisible assistant" in paint and ink formulations, and an efficient plasticizer in the rubber and plastics industries. According to the "like dissolves like" principle, the oxygen atom provides weak polarity, while the long alkyl chain ensures non-polar dissolving power.

Stable Chemical Properties
Strong oxidation resistance: It is less prone to forming explosive peroxides compared to diethyl ether.
Acid-base tolerance: It shows excellent stability within the pH range of 2-12.
Low water solubility: Its low water solubility (0.3g/100mL of water) makes it particularly suitable for liquid-liquid extraction and separation.
Process-Friendly Characteristics
Moderate boiling point (142°C): This makes it easy to recover by distillation.
Low surface tension (25.4 mN/m): This property helps with the wetting of porous materials.
Slow evaporation rate: With an evaporation rate of 0.2 (when diethyl ether = 1), it is suitable for coating processes that require long operating times.
A Full Picture of Application Scenarios
| Field | Typical Use | Irreplaceability |
| Pharmaceutical | Solvent for antibiotic crystallization | Low toxicity, compliant with GMP standards |
| Electronics | Component in semiconductor cleaning agents | No metal residues, high purity |
| Coatings | Epoxy resin thinner | Improves leveling, reduces bubble defects |
| Scientific Research | Solvent for Grignard reactions | Safer and has a higher boiling point than diethyl ether |
Safety Usage Guide
Although Dibutyl Ether is relatively safe and classified as a low-toxicity substance, caution is still required.
Ventilation Protection: Vapors may cause dizziness, so forced ventilation is required in operating rooms.
Static Electricity Prevention: Its resistivity is as high as 1 × 10¹³ Ω·cm, so grounding is necessary for explosion prevention.
Storage Essentials: It is not corrosive to metals and can be stored in containers made of steel, copper, or aluminum. It is prone to forming explosive peroxides during storage, which must be removed before distillation. Light can promote peroxide formation, so it should be sealed and stored in a cool place. Nitrogen protection is recommended to prevent peroxide accumulation (the suggested addition of 0.005% BHT antioxidant).
Key Purchasing Indicators
Purity: ≥99.5% (GC detection)
Moisture: ≤0.05% (Karl Fischer method)
Peroxides: ≤10ppm (Potassium iodide test)
In conclusion, Dibutyl Ether is like the "Swiss Army knife" of the chemical world. It lacks extreme characteristics but demonstrates balanced strength in multiple fields. Whether for precise synthesis in the laboratory or for large-scale production in a factory, it can reliably safeguard your processes with its stable performance.
Looking for a reliable bulk supplier of Dibutyl Ether (CAS 142-96-1)?
Aure Chemical provides Premium Dibutyl Ether (CAS 142-96-1) raw materials.
View our Dibutyl Ether (CAS 142-96-1) product page