What is Dichlorodimethylsilane (CAS 75-78-5)?
Dichlorodimethylsilane (DDMS) is an organosilicon compound, specifically a chlorosilane with the molecular formula C₂H₆Cl₂Si, commonly represented as (CH₃)₂SiCl₂. It serves as a versatile intermediate in the synthesis of silicon-based materials, particularly silicones. This compound is crucial in the chemical industry due to its role as a key building block for producing silicone polymers, which are widely used in various industrial applications for their thermal stability, water repellency, and flexibility. The CAS number for dichlorodimethylsilane is 75-78-5, and it is also known by synonyms such as dimethyldichlorosilane and DDMS.
Chemical Identity and Structure
Dichlorodimethylsilane has the molecular formula C₂H₆Cl₂Si and a molecular weight of 129.06 g/mol. Its structure consists of 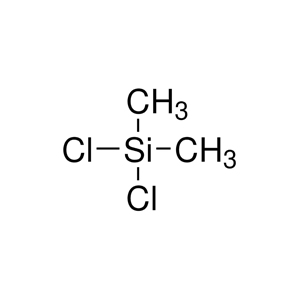 a central silicon atom bonded to two methyl groups (CH₃) and two chlorine atoms (Cl), forming a tetrahedral geometry with bond angles of approximately 109.5 degrees, resulting in a three-dimensional pyramid shape. The compound is also referred to by synonyms including dimethyldichlorosilane, dichlorodimethylsilicon, methyl dichlorosilane, and silicon dichlorodimethyl. In terms of appearance, it is a colorless to brown liquid with a sharp, pungent odor reminiscent of hydrochloric acid or other chlorinated compounds, and it emits smoke when exposed to humid air due to its reactivity with moisture.
a central silicon atom bonded to two methyl groups (CH₃) and two chlorine atoms (Cl), forming a tetrahedral geometry with bond angles of approximately 109.5 degrees, resulting in a three-dimensional pyramid shape. The compound is also referred to by synonyms including dimethyldichlorosilane, dichlorodimethylsilicon, methyl dichlorosilane, and silicon dichlorodimethyl. In terms of appearance, it is a colorless to brown liquid with a sharp, pungent odor reminiscent of hydrochloric acid or other chlorinated compounds, and it emits smoke when exposed to humid air due to its reactivity with moisture.
Physical and Chemical Properties
Dichlorodimethylsilane exhibits several key physical properties that make it suitable for industrial handling and processing. Its melting point is -76 °C, boiling point is 70 °C (lit.), and density is 1.333 g/mL at 20 °C. The refractive index is n20/D 1.500, and it has a vapor pressure of less than 200 hPa at 20 °C. It has a flash point of 3 °F and an autoignition temperature of 425 °C, with explosive limits ranging from 1.75% to 48.5% (V). The compound is moisture-sensitive and reacts with water, showing hydrolytic sensitivity rated at 8 (reacts rapidly with moisture, water, or protic solvents). It is soluble in chlorinated and ethereal solvents but reacts violently with protic solvents like water and alcohols.
Chemically, dichlorodimethylsilane is highly reactive, particularly undergoing hydrolysis where it reacts vigorously with water to produce hydrochloric acid (HCl) and silanols, such as dimethylsilanediol. This reaction is exothermic and can release heat. It is stable under inert conditions but incompatible with strong oxidizing agents, caustics, and ammonia, and it is highly flammable. Handling considerations include storing it below +30 °C in a dry, well-ventilated area away from moisture to prevent unintended reactions.
Production Routes of Dichlorodimethylsilane
1.Müller–Rochow process
The primary industrial production method for dichlorodimethylsilane is the Müller–Rochow process, also known as the Direct Process. This involves reacting methyl chloride (CH₃Cl) with silicon metal in the presence of a copper catalyst (such as Cu₂O or copper(I) chloride) at temperatures between 250–300 °C. The reaction generates a mixture of methylchlorosilanes, including monomethyltrichlorosilane (CH₃SiCl₃), dimethyldichlorosilane ((CH₃)₂SiCl₂), trimethylchlorosilane ((CH₃)₃SiCl), and methyldichlorosilane (CH₃SiHCl₂). These products are then separated through fractional distillation based on their differing boiling points.
Reaction Principle
In the presence of a copper-based catalyst, methyl chloride (CH₃Cl) reacts directly with elemental silicon (Si) to form a mixture of methylchlorosilanes. The global reaction can be written as:
n CH₃Cl + Si → (CH₃)ₙSiCl₄₋ₙ (n = 1, 2, 3)
Main Products & Representative Equations
Dichlorodimethylsilane (target product)
2 CH₃Cl + Si → (CH₃)₂SiCl₂
Methyltrichlorosilane
CH₃Cl + Si → CH₃SiCl₃
Trimethylchlorosilane
3 CH₃Cl + Si → (CH₃)₃SiCl
Product Distribution & Separation
Dichlorodimethylsilane ((CH₃)₂SiCl₂) is typically the major component in the product mixture; selectivity can be optimized (often reported in the ~70–90% range) depending on catalyst formulation and operating conditions.
Other methylchlorosilanes (e.g., CH₃SiCl₃, (CH₃)₃SiCl) are valuable co-products and are separated by fractional distillation.
Key Notes
Process performance (conversion, selectivity, lifetime) is highly sensitive to catalyst composition, reactor temperature, methyl chloride/Si ratio, and promoters.
Downstream purification ensures on-spec DDMS for use as a core organosilicon intermediate in silicones, coatings, sealants, and specialty chemicals.
2.Grignard reaction
An alternative laboratory-scale method is the Grignard reaction, where methyl chloride reacts with silicon tetrachloride in the presence of magnesium. On an industrial scale, this process is carried out in large reactors under strict control of temperature and pressure to ensure safety and efficiency, given the compound's reactivity. Globally, production is dominated by major chemical manufacturers, with dichlorodimethylsilane being a high-volume intermediate in the silicone industry.
Formation of Grignard Reagents
Grignard reagents have the general formula R–Mg–X (R = alkyl or aryl; X = halide: Cl, Br, I). They are prepared by reacting an organohalide with magnesium metal in dry ether.
R–X + Mg —(dry ether)→ R–Mg–X
Example:
CH3Br + Mg —(ether)→ CH3–Mg–Br
Addition to Carbonyl Compounds (General Patterns)
Grignard reagents add to carbonyl compounds; after acidic workup (H3O+), the corresponding alcohol is obtained.
With Formaldehyde (HCHO)
R–Mg–X + HCHO —(H3O+)→ R–CH2–OH
Product: primary alcohol.
With Other Aldehydes (R'CHO)
R–Mg–X + R'–CHO —(H3O+)→ R–CH(OH)–R'
Product: secondary alcohol.
With Ketones (R'–CO–R'')
R–Mg–X + R'–CO–R'' —(H3O+)→ R–C(OH)–R'–R''
Product: tertiary alcohol.
With Carbon Dioxide (CO2)
R–Mg–X + CO2 —(H3O+)→ R–COOH
Product: carboxylic acid.
Other Common Reactions
With Epoxides
Grignard reagents open epoxide rings to give alcohols with chain extension (after acidic workup):
R–Mg–X + (epoxide) —(H3O+)→ R–CH2–CH2–OH
With Nitriles (R'–C≡N)
After addition and hydrolysis, nitriles yield ketones:
R–Mg–X + R'–C≡N —(H3O+)→ R–C(=O)–R'
With Esters (R'–COOR'')
Esters react with two equivalents of Grignard reagent to give tertiary alcohols after workup:
R–Mg–X + R'–COOR'' —(2 equiv; H3O+)→ R2C(OH)–R'
Summary Table
| Reaction Partner | Representative Equation | Typical Product (after H3O+) |
| Formaldehyde (HCHO) | R–Mg–X + HCHO → R–CH2–OH | Primary alcohol |
| Aldehyde (R'CHO) | R–Mg–X + R'–CHO → R–CH(OH)–R' | Secondary alcohol |
| Ketone (R'–CO–R'') | R–Mg–X + R'–CO–R'' → R–C(OH)–R'–R'' | Tertiary alcohol |
| Carbon dioxide (CO2) | R–Mg–X + CO2 → R–COOH | Carboxylic acid |
| Epoxide | R–Mg–X + epoxide → R–CH2–CH2–OH | Alcohol (chain extended) |
| Nitrile (R'–C≡N) | R–Mg–X + R'–C≡N → R–C(=O)–R' | Ketone |
| Ester (R'–COOR'') | 2 R–Mg–X + R'–COOR'' → R2C(OH)–R' | Tertiary alcohol |
Practical Notes
Grignard reagents are extremely water-sensitive. All glassware and solvents must be dry; reaction solvents are typically anhydrous diethyl ether or THF.
They react with protic sources (water, alcohols, NH acids) and are quenched by them—acidic workup is required to obtain neutral organic products.
Choice of R and halide (Br, I more reactive than Cl) and reaction temperature influence formation rate and reactivity.
Applications of Dichlorodimethylsilane
Dichlorodimethylsilane is primarily used as a precursor in the production of silicones, including silicone oils, elastomers, and resins. Through hydrolysis, it forms linear and cyclic siloxanes, which are polymerized into silicone products used in lubricants, sealants, medical devices, cookware, and more. It is also employed in coatings, adhesives, and sealants for its ability to enhance material properties like hydrophobicity and durability.
As an intermediate in specialty chemicals, it is utilized in the synthesis of polysilanes (precursors to silicon carbide), optically active ansa-metallocene polymerization catalysts, and resin-bound siloxanes. Additional applications include surface modification, such as coating glass to prevent micro-particle adsorption, acting as a silane coupling agent in silica nanoparticle synthesis, and serving as a reagent in organic reactions like pinacol cyclization, protecting groups for diols and carbonyls, and hydrogenation catalysis.
Safety and Handling Overview
Dichlorodimethylsilane is both flammable and corrosive, posing significant hazards if not handled properly. It hydrolyzes rapidly upon contact with water or moisture, releasing hydrochloric acid gas and heat, which can cause severe burns, eye damage, and respiratory irritation. It is highly flammable, with vapors capable of forming explosive mixtures, and may cause drowsiness, dizziness, or damage to organs through prolonged exposure.
Safety guidelines include wearing protective equipment such as gloves, eye protection, and chemical-resistant clothing, ensuring adequate ventilation to prevent inhalation, and storing in tightly sealed containers away from heat, sparks, water, and incompatible materials. In case of exposure, rinse affected areas with water (except if swallowed, where medical attention is required without inducing vomiting), and dispose of as hazardous waste to avoid environmental harm, as it is toxic to aquatic life. Refer to the Safety Data Sheet (SDS) for detailed protocols.
Dichlorodimethylsilane (CAS 75-78-5) is a fundamental organosilicon intermediate defined by its (CH₃)₂SiCl₂ structure and role in facilitating the creation of advanced materials through its reactivity and versatility. As an essential component in the silicone industry, it underpins the production of durable, high-performance products across sectors like manufacturing, healthcare, and electronics. For those in research or industry, exploring further resources such as Safety Data Sheets or supplier technical details can provide deeper insights into its safe and effective utilization.
Related Articles
Looking for a reliable bulk supplier of Dichlorodimethylsilane?
Aure Chemical provides Premium Dichlorodimethylsilane (DDMS) raw materials.
View our Dichlorodimethylsilane (DDMS) product page
