An Overview of Trifluoroacetic Acid (CAS 76-05-1)
What is Trifluoroacetic Acid? Trifluoroacetic acid, often abbreviated as TFA, is a synthetic organofluorine compound widely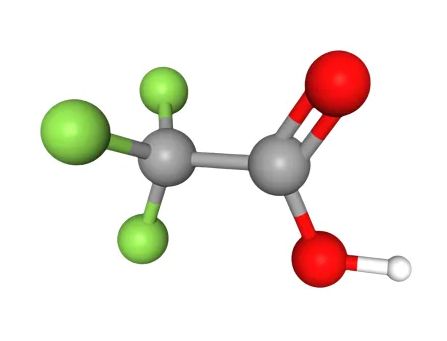 recognized for its role in organic chemistry and various industrial applications. It is a colorless liquid with a pungent, vinegar-like odor, and its chemical formula is C₂HF₃O₂, more commonly written as CF₃COOH. This compound is a derivative of acetic acid where the three hydrogen atoms in the methyl group are replaced by fluorine atoms, making it a member of the haloacetic acid family.
recognized for its role in organic chemistry and various industrial applications. It is a colorless liquid with a pungent, vinegar-like odor, and its chemical formula is C₂HF₃O₂, more commonly written as CF₃COOH. This compound is a derivative of acetic acid where the three hydrogen atoms in the methyl group are replaced by fluorine atoms, making it a member of the haloacetic acid family.
Chemical Properties of TFA
Trifluoroacetic acid's molecular structure closely resembles that of acetic acid (CH₃COOH), but the substitution of fluorine atoms in the trifluoromethyl group (CF₃) imparts unique properties. This structural modification enhances its acidity and alters its physical characteristics.
The trifluoroacetic acid density is approximately 1.489 g/cm³ at 20°C, which is higher than that of water (1.0 g/cm³) and acetic acid (1.049 g/cm³ at 20°C), reflecting the density contribution from the heavy fluorine atoms. In laboratory settings, when handling a sample of trifluoroacetic acid C₂HF₃O₂, this density value is crucial for accurate volume-to-mass conversions during experiments.
The trifluoroacetic acid boiling point is 72.4°C, significantly lower than that of acetic acid, which boils at 118°C. This lower boiling point facilitates easier removal of TFA from reaction mixtures through evaporation, making it advantageous in synthetic processes where volatility is desired.
Regarding its acidity, the trifluoroacetic acid pKa is approximately 0.23, indicating it is a much stronger acid than typical carboxylic acids like acetic acid (pKa 4.76). (Note: Some sources report a pKa of 0.52, but the commonly cited value in organic chemistry contexts is 0.23, reflecting its high acid strength.)
Acidity and Classification
Is trifluoroacetic acid a strong acid? Yes, trifluoroacetic acid is considered a strong acid in the context of organic acids, with its low pKa value (around 0.23) signifying near-complete dissociation in aqueous solutions. While not as strong as mineral acids like hydrochloric acid (pKa -6.3), it is exceptionally potent among carboxylic acids.
Why is trifluoroacetic acid stronger than acetic acid? The enhanced acidity stems from the electron-withdrawing effect of the three fluorine atoms in the CF₃ group. These highly electronegative atoms pull electron density away from the carboxylic acid group through inductive effects, weakening the O-H bond and stabilizing the conjugate base (trifluoroacetate ion). This results in a Ka value about 34,000 times higher than that of acetic acid.
Is trifluoroacetic acid organic or inorganic? Trifluoroacetic acid is classified as an organic acid because it contains carbon-hydrogen bonds and is derived from acetic acid, a classic organic compound. Despite the presence of fluorine, it falls under organofluorine chemistry rather than inorganic acids like sulfuric acid.
What is Trifluoroacetic Acid used for
Trifluoroacetic acid has diverse applications across scientific and industrial fields due to its strong acidity, volatility, and solubility in organic solvents.
In peptide and protein chemistry, TFA is essential for cleaving protecting groups, such as the tert-butoxycarbonyl (Boc) group, during solid-phase peptide synthesis. It is also used as an ion-pairing agent in high-performance liquid chromatography (HPLC) for separating peptides and small proteins.
As a solvent or reagent in organic synthesis, TFA serves in reactions requiring acidic conditions without oxidation, and it is a precursor to derivatives like trifluoroacetic anhydride and trifluoroperacetic acid. In the pharmaceutical industry, it acts as a catalyst and raw material for synthesizing drugs, while in the polymer sector, it aids in producing fluoropolymers. Additionally, TFA is employed as a solvent in NMR spectroscopy, a calibrant in mass spectrometry, and in the production of agricultural chemicals.
Safety and Handling
 Trifluoroacetic acid is highly corrosive and toxic, requiring careful handling to avoid severe health risks. Contact with skin or eyes can cause severe burns that heal poorly and may lead to necrosis, while inhalation may result in respiratory irritation, coughing, shortness of breath, and potential pulmonary edema. It has demonstrated liver and reproductive toxicity in animal studies, with recent proposals to classify it as reproductively toxic. The LC50 for rats is 10.01 mg/L over 4 hours, indicating moderate inhalation toxicity.
Trifluoroacetic acid is highly corrosive and toxic, requiring careful handling to avoid severe health risks. Contact with skin or eyes can cause severe burns that heal poorly and may lead to necrosis, while inhalation may result in respiratory irritation, coughing, shortness of breath, and potential pulmonary edema. It has demonstrated liver and reproductive toxicity in animal studies, with recent proposals to classify it as reproductively toxic. The LC50 for rats is 10.01 mg/L over 4 hours, indicating moderate inhalation toxicity.
For proper handling, use in well-ventilated areas or fume hoods, wear protective equipment including gloves, goggles, and respirators, and avoid contact with skin or inhalation of vapors. Store in tightly sealed containers made of compatible materials like glass or PTFE, away from bases, metals, and reducing agents, as it can react vigorously.
Environmentally, TFA is a persistent "forever chemical" classified as a per- and polyfluoroalkyl substance (PFAS), accumulating in water, soil, food, and human bodies. It degrades slowly and has been linked to increasing concentrations in global water sources, posing risks to ecosystems and human health. Removal from water requires methods like reverse osmosis, which are costly.
Conclusion
In summary, trifluoroacetic acid is a powerful organic acid with the formula C₂HF₃O₂, distinguished by its low pKa, high density, and relatively low boiling point compared to acetic acid. Its strength as an acid, driven by the electron-withdrawing fluorine atoms, makes it invaluable in applications ranging from peptide synthesis to pharmaceutical manufacturing. However, its corrosiveness, toxicity, and environmental persistence underscore the need for cautious use. What is trifluoroacetic acid if not a double-edged sword in modern chemistry—essential yet demanding respect for its hazards? As research continues, balancing its industrial importance with safety and sustainability remains key.

