Exploring Acrylic Acid: Uses, Production, Safety, and Where to Buy
What is Acrylic Acid
Acrylic Acid (CAS 79-10-7) is a colorless organic compound that serves as a fundamental building block in various industrial applications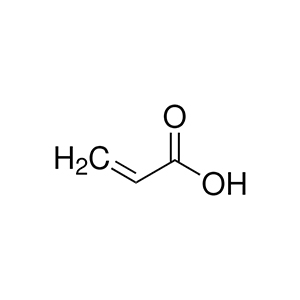 due to its versatility and reactivity. The formula of acrylic acid is C₃H₄O₂, often written as CH₂=CHCOOH, classifying it as an unsaturated carboxylic acid. It belongs to the same class as other well-known compounds like citric acid and benzoic acid. Unlike many carboxylic acids, acrylic acid is not highly reactive at room temperature under normal conditions, but it is extremely prone to polymerization, where molecules link together to form long chains known as polyacrylates. To prevent premature polymerization during storage and transportation, it is typically stabilized with additives such as hydroquinone or other inhibitors.
due to its versatility and reactivity. The formula of acrylic acid is C₃H₄O₂, often written as CH₂=CHCOOH, classifying it as an unsaturated carboxylic acid. It belongs to the same class as other well-known compounds like citric acid and benzoic acid. Unlike many carboxylic acids, acrylic acid is not highly reactive at room temperature under normal conditions, but it is extremely prone to polymerization, where molecules link together to form long chains known as polyacrylates. To prevent premature polymerization during storage and transportation, it is typically stabilized with additives such as hydroquinone or other inhibitors.
The acrylic acid structure features a carbon-carbon double bond adjacent to a carboxyl group (-COOH), which contributes to its reactivity in forming polymers and derivatives. This structure includes a vinyl group (CH₂=CH-) attached to the carboxylic acid, making it an alpha,beta-unsaturated acid. The molecular weight of acrylic acid is approximately 72.06 g/mol, calculated from its atomic composition: three carbon atoms (36 g/mol), four hydrogen atoms (4 g/mol), and two oxygen atoms (32 g/mol).
In terms of physical properties, acrylic acid appears as a clear, corrosive liquid with a pungent, acrid odor reminiscent of vinegar. Its density is about 1.051 g/mL at 20°C, making it slightly denser than water. The acrylic acid boiling point (also referred to as the boiling point of acrylic acid) is 141°C (286°F), while the melting point is around 13-14°C (55-57°F), allowing it to solidify at temperatures just above freezing. The acrylic acid flash point is approximately 54°C (130°F) in open cup tests, indicating it is flammable and requires careful handling to avoid ignition sources.
How to Make Acrylic Acid
The production of acrylic acid involves several chemical processes, with industrial methods focusing on efficiency and scalability. The primary method is the oxidation of propylene, where propylene gas (C₃H₆) reacts with oxygen in the presence of catalysts, such as transition metals (e.g., molybdenum or bismuth-based) or biocatalysts, to yield acrylic acid. This two-step process first produces acrolein as an intermediate before further oxidation. It is the most widely used industrial route, accounting for over 90% of global production due to its cost-effectiveness and high yields.
Alternative methods include:
Acrylate hydration: Acrylate esters react with water under acidic conditions (e.g., using chromium trioxide or phosphoric acid) to form an alcohol intermediate, which is then oxidized and dehydrated to acrylic acid.
Acrylonitrile hydrogenation: Acrylonitrile (CH₂=CHCN) is hydrogenated with water over a catalyst to produce acrylamide, followed by hydrolysis to acrylic acid.
Oxidation of acrylonitrile: Acrylonitrile is oxidized to form an oxide intermediate, which is hydrolyzed to acrylic acid.
These methods are less common but useful for specific applications or when raw materials like acrylonitrile are readily available. Globally, production capacity exceeds 8 million tons annually, with advancements in catalysis improving energy efficiency and reducing environmental impact.
What is Acrylic Acid Used For?
Acrylic acid's versatility stems from its ability to polymerize and form derivatives with desirable properties. It is a key precursor in manufacturing acrylic esters, which are polymerized into materials used in paints, adhesives, coatings, and plastics. These polymers offer excellent UV resistance, solvent resistance, and strong covalent bonds, enhancing durability and performance in products like resins and thermoplastics.
Beyond polymers, acrylic acid derivatives play roles in everyday items. For instance, it contributes to detergents as a builder for water softening, pulp processing agents for paper production, and even food additives like calcium stearoyl lactylate, a natural emulsifier that maintains texture in dairy products such as cheese spreads. Its functional groups—carbonyl (-CO-) and hydroxyl (-OH)—enable strong interactions with polar molecules, making it ideal for surfactants and emulsifiers.
Additional benefits include:
Adhesion and durability: Bonds well to surfaces like metal, glass, plastic, ceramic, and concrete, maintaining properties in harsh environments.
Chemical resistance: Resists corrosion from most chemicals.
Water and moisture resistance: Performs well in humid conditions.
Rapid curing: Speeds up production processes.
Low volatility: Minimizes harmful emissions during use.
Wide compatibility: Suitable for industries including construction, automotive, aerospace, electronics, healthcare, and household goods.
Decorative properties: Offers color and texture options for applications like sports field markings.
In superabsorbent polymers (SAPs), acrylic acid is cross-linked to create materials that absorb hundreds of times their weight in water, used in diapers, sanitary products, and agriculture for soil moisture retention.

Is Acrylic Acid Safe for Skin?
No, acrylic acid is not safe for skin. It is classified as a strong irritant and corrosive substance that can cause severe burns, redness, blistering, and long-term damage upon contact. Direct exposure may lead to chemical burns due to its acidic nature (pH around 2-3) and ability to penetrate skin layers. Inhalation or eye contact can also result in irritation, respiratory issues, or blindness. Prolonged or repeated exposure might cause sensitization or allergic reactions in sensitive individuals.
Safety guidelines from organizations like the EPA and OSHA recommend avoiding skin contact entirely. When handling, use personal protective equipment (PPE) such as chemical-resistant gloves, goggles, and protective clothing. In case of exposure, rinse immediately with water for at least 15-20 minutes and seek medical attention. While derivatives like polyacrylic acid in cosmetics are generally safer at low concentrations, pure acrylic acid should never be applied to skin.
Acrylic Acid in China: Production and Suppliers
China is a dominant player in the global acrylic acid market, producing over 40% of the world's supply, driven by its vast petrochemical industry and access to raw materials like propylene. Major production hubs include Shandong, Jiangsu, and Shanghai provinces, where facilities leverage advanced oxidation technologies for high-output manufacturing. In 2025, China's acrylic acid capacity is estimated at around 3-4 million tons annually, supporting exports to Europe, North America, and Asia. Environmental regulations have pushed producers toward greener processes, reducing emissions and improving sustainability.
Where to Buy Acrylic Acid and Current Pricing
For those wondering where to buy acrylic acid, it is available from specialized chemical distributors and online platforms, primarily for industrial or laboratory use.
Online purchases require compliance with hazardous material shipping regulations, and buyers should verify purity (typically 98-99.5%) and stabilization.
Aure Chemical is dedicated to providing superior chemical solutions and unmatched customer support. By partnering with us for your Stabilized Acrylic Acid requirements.
Choose Aure Chemical for a trustworthy and dependable supply of high-quality Stabilized Acrylic Acid. We are ready to support your most complex and innovative chemical projects.
As for acrylic acid price, it fluctuates based on market demand, raw material costs (e.g., propylene), and global supply chains. In August 2025, prices in China average around 6,200-6,750 RMB per metric ton (approximately 860-940 USD/ton), reflecting a bearish trend due to sufficient supply and weak downstream demand. Globally, spot prices range from 1,100-1,300 USD per ton, with esters of acrylic acid exporting at about 2,153 USD/ton in some regions. The overall market is projected to grow from USD 11.3 billion in 2023 to USD 13.8 billion by later years, but individual purchases for lab-grade may cost $80-140 per liter, depending on quantity and supplier.
In summary, acrylic acid is an essential chemical with broad industrial significance, but it demands careful handling due to its hazards. For procurement or further details, consult certified suppliers and stay updated on market trends.

